Abstract
Daratumumab is a human IgG1k monoclonal antibody targeting the CD38 surface antigen on plasma cells, and has proven efficacy in multiple myeloma. AL amyloidosis is a plasma cell disorder in which clonal bone marrow plasma cells produce immunoglobulin light chains that misfold into fibrils that are deposited in organs, leading to organ dysfunction and failure. While the biology of the clonal plasma cell in AL amyloidosis is distinct from that of myeloma, clonal plasma cells in AL amyloidosis do express surface CD38, providing a rationale for the use of daratumumab in AL amyloidosis. Infusion reactions (IR) of 48% were reported in patients who received daratumumab as monotherapy for relapsed multiple myeloma. Therefore, we designed a clinical trial to study tolerability of daratumumab in patients with relapsed AL amyloidosis (clinicaltrials.gov identifier: NCT02841033). The primary objective was to determine the safety and tolerability of infusion of daratumumab, with respect to IR. The secondary objectives were to assess hematologic response, clinical response and time to next treatment.
Accrual on this clinical trial started in April 2017. Patients with relapsed AL amyloidosis after ≥1 prior therapy, and involvement of at least one major vital organ , eGFR of >20 mL/min, AST/ALT < 3xULN, NTproBNP <8500 pg/mL, LVEF >30%, FEV1 >50% in patients with known COPD or chronic smokers, and ECOG performance status of <3, received daratumumab at 16 mg/kg IV infusion weekly for weeks 1-8, followed by every 2 weeks for weeks 9-24 and every 4 weeks thereafter until progression or unacceptable toxicities, for up to 24 months. The first infusion of daratumumab was administered in 1000 mL, the second infusion in 500 mL if no grade 1 or greater reactions occurred throughout the first 3 hours of the first infusion, and subsequent doses were administered in 500 mL of saline. All patients received acetaminophen, diphenhydramine, loratidine, famotidine, monteleukast and methyprednisolone (100 mg for the first 2 infusions and 60 mg thereafter) 30-60 minutes prior to infusion. Ondansetron was added after developement of grade 1 nausea/vomiting in the first 2 patients. Diphenhydramine, famotidine and methyprednisolone (40 mg) were also administered 2 hours after the start of infusion during the first 2 infusions even if there was no reaction. Methylprednisolone 20 mg (or its equivalent) and monteleukast were adminsitered 24 and 48 hours after start of infusion for the first 2 infusions and then monteleukast was optional. All patients received prophylaxis with acyclovir.
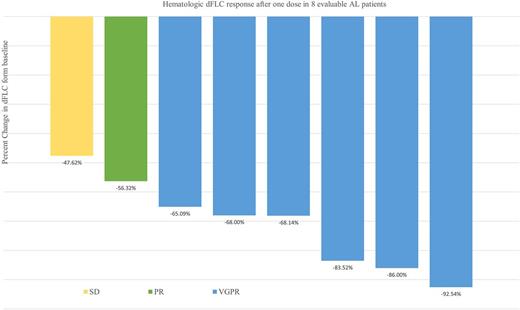
At data cut-off (July 23, 2017) 8 patients have been enrolled. The median age was 63 years (range, 60-83), and median number of prior therapies was 3 (range, 1-6): 6 (75%) had received HDM/SCT, 5 (62.5%) had received immunomodulatory agents, and 7 (87.5%) proteosome inhibitors. Three patients were refractory to prior therapy and the median time from last therapy was 15 months. The median time from diagnosis to enrollment on this trial was 68 months. The median number of organ systems involved was 2 (range, 1-4). Five patients (62.5%) had involvement of >2 organ systems. All 8 (100%) patients had cardiac biomarker stage II or III disease. Median NT-proBNP level was 1650.5 pg/mL (range, 656-3962) and urine protein excretion was 0.41 g/24 h (range, 0-10.1), while median dFLC was 196.8 mg/L (range, 20.4-854). At the time of this report, 7 patients continue on study and a total of 65 infusions have been completed. The median number of infusions received per patient is 9 (range, 2-12). One patient was removed from the protocol after developing progression of plasma cell dyscrasia markers after 2 cycles. No patient experienced a grade 3-4 IR. Only 2 patients experienced grade 1 nausea and vomiting during first infusion, which resolved after an antiemetic, without experiencing IR. There was no interruption or delay of infusion. No hospitalization for IR was required. The median time of first infusion was 6.5 hours and 2nd infusion was 4.2 hours. Hematologic responses were rapid and are shown in Figure 1.
Daratumumab infusion is well tolerated in patients with relapsed AL amyloidosis, when administered with appropriate pre and post-medications. Infusion related reactions were minimal and not comparable to reported incidence in myeloma. Preliminary data suggest a rapid hematologic response after 1 dose of daratumumab in patients with AL amyloidosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal